Supported by

For this supplement, the expert faculty provided detailed perspectives based on their extensive experience with the management of cancer patients with bone involvement, especially skeletal-related events associated with solid tumor metastases and lytic lesions due to multiple myeloma.

Beth Faiman, PhD, MSN, APRN-BC, AOCN, FAAN
Nurse Practitioner
Department of Hematology and Medical Oncology
Cleveland Clinic
Cleveland, OH

Kathleen Colson, RN, BSN, BS
Clinical Research Nurse
Dana-Farber Cancer Institute (Retired January 2021)
Boston, MA

Jonathan Kaufman, MD
Associate Professor
Director (Interim), Division of Hematology
Department of Hematology and Medical Oncology
Section Chief, Ambulatory Infusion Services
Winship Cancer Institute
Emory University School of Medicine
Atlanta, GA

Tiffany Richards, PhD, MS, APN, AOCNP
Supervisor, Myeloma Advanced Practice Providers
Department of Lymphoma/Myeloma
MD Anderson Cancer Institute
Houston, TX
Introduction
In healthy individuals, bones are consistently remodeled through cycles of breakdown and synthesis to respond to environmental influences such as microfractures or mechanical forces.1
This process allows the skeleton to adjust appropriately to environmental pressure and is executed through tightly controlled and coordinated mechanisms of breakdown and synthesis.1 In patients with cancer, bone is a common site of metastasis.2 Skeletal-related events (SREs) secondary to metastases from solid tumors or lytic lesions from multiple myeloma (MM) are common among patients living with cancer.3-5 The presence of lytic lesions or cancer metastases to bone and the mode of interference of cancer cells with normal bone turnover have distinct pathophysiologic consequences across cancer types.6 This supplement will provide an overview of SREs related to solid tumor metastases and lytic lesions due to MM, including their negative impact on patients and the healthcare system, as well as provide expert faculty perspectives on the management of cancer patients with bone involvement.
Incidence of Bone Metastases, Lytic Lesions, and SREs
Unfortunately, bone is a common site of cancer metastasis.2 In an analysis of US electronic medical records, the cumulative incidence of bone metastasis from cancer was 2.9% at 30 days, 4.8% at 1 year, 5.6% at 2 years, 6.9% at 5 years, and 8.4% at 10 years.7 While most cancer types have the potential to metastasize to bone, solid tumors from prostate, lung, kidney, and breast cancers do so most often.7 The incidence of bone metastases at 1 year is 18% in patients with prostate cancer, 10.4% in lung cancer, 5.8% in kidney cancer, and 3.4% in breast cancer. At 5 years, these incidences rise to 24.5%, 12.4%, 8.4%, and 6.0%, respectively.7 In patients with advanced metastatic disease, the relative incidence of bone metastasis by type of tumor is estimated at 65% to 75% in breast cancer, 65% to 75% in prostate cancer, 60% in thyroid cancer, 30% to 40% in lung cancer, 40% in bladder cancer, 20% to 25% in renal-cell carcinoma, and 14% to 45% in melanoma.8 The vertebrae, pelvis, and thoracic bones are common sites of metastasis among patients with lung, prostate, and breast cancers.9,10 In addition to those with solid tumors, bone destruction is extremely common in patients with MM of any stage; a striking 80% to 90% of patients with MM will develop osteolytic bone lesions—a “punched-out” area of severe bone loss.11
Infiltrating metastatic cells and cancer pathology can offset the delicate balance of normal bone physiology, which weakens and damages bone tissue, putting patients at risk for SREs.12-14 SREs are defined as pathological fractures, need for radiation therapy to bone, surgery to bone, and spinal cord compression.15 Bone metastases from solid tumors and lytic lesions due to MM, along with resultant SREs, can cause significant pain and decrease quality of life (QOL).16-18 Regardless of the source of the bone metastasis or lesion, when a patient’s cancer affects their bones it places a significant burden on the patient and the healthcare system. It is important for healthcare providers to be aware of this burden to aid their recognition of the signs and consequences of bone metastases and lesions and to inform their interactions with patients.
Overview of Bone Physiology in Cancer
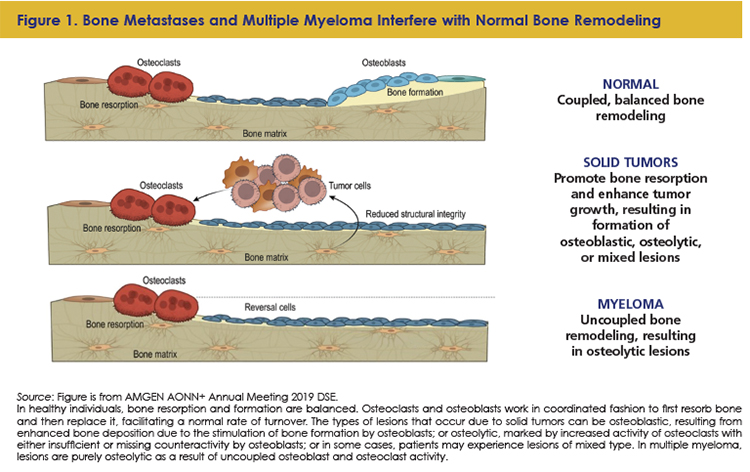
The pathophysiology of cancer-related bone involvement varies with tumor type, but in all cases, bone lesions result from disruptions to the body’s natural processes of bone turnover (Figure 1).18-22 In healthy individuals, bone resorption and formation are balanced.18 Osteoclasts and osteoblasts work in coordinated fashion to first resorb bone and then replace it, facilitating a normal rate of turnover.18 Osteoclasts adhere to the bone surface and secrete bone-dissolving proteases, releasing bone mineral to the extracellular space where it is resorbed.18 In concert, osteoblasts, stimulated by parathyroid hormone, prostaglandins, and cytokines, promote the synthesis of bone matrix.18
Depending on the underlying pathology of the cancer-related bone involvement, bone is either destroyed or synthesized inappropriately, which causes lesions that result in a loss of structural integrity and may lead to complications that affect the patient’s QOL.6 The types of lesions that occur due to solid tumors can be osteoblastic, resulting from enhanced bone deposition due to the stimulation of bone formation by osteoblasts; or osteolytic, marked by increased activity of osteoclasts with either insufficient or missing counteractivity by osteoblasts; or in some cases, patients may experience lesions of mixed type.6
In patients with solid tumors, bone metastases stimulate bone resorption, which releases growth factors, reduces bone structural integrity, and can cause hypercalcemia.13,18 In addition, the bone microenvironment nurtures tumor cells, and the involvement of tumor in bone and resulting lesions can lead to or exacerbate pain.18,23 In MM, lesions are purely osteolytic as a result of uncoupled osteoblast and osteoclast activity.11,20
Can you briefly describe the pathophysiology of SREs related to solid tumors and MM?
Jonathan Kaufman, MD: “What is unique about the pathophysiology of myeloma bone disease is that myeloma is one of the few bone diseases in cancer that is a purely lytic bone disease. It is purely lytic in 2 processes, one by activation of the osteoclasts, two by active suppression of the osteoblasts. With that, you get a pure lytic bone lesion.
In contrast, the more common solid tumors that are associated with bone metastases, like lung cancer, breast cancer, and prostate cancer, are going to be a mixed osteolytic and osteoblastic, where you have activation of both the osteoclasts as well as the osteoblasts.”
How do SREs manifest in patients?
Beth Faiman, PhD, MSN, APRN-BC, AOCN, FAAN: “In terms of the clinical presentation, let’s think of that breast cancer patient, they’re just striding along, and they’ve completed their therapy, they’re on their maintenance hormonal therapy and then they developed back or bone pain. That’s how I see people with solid tumors presenting, not at the very beginning of the disease as much as it does later on with the metastatic disease, although you can get a mix and have it at presentation.”
The incidence of SREs is high among patients with bone metastases and those with MM. In a retrospective analysis of treatment-naïve patients with bone metastases from solid tumors,24 by cancer type, the mean number of SREs per year in patients with metastatic breast cancer is 4, for patients with prostate cancer it is 1.5, and for those with lung and other solid tumors, it is 2.7.3-5 In patients receiving antiresorptive treatment with bone targeting agents (BTAs), the mean number of SREs per year drops to 2.5, 0.8, and 1.7, in patients with breast, prostate, and lung and other solid tumors, respectively.3-5 For patients with MM, without BTA treatment, the mean number of SREs per year is 2.2. With BTAs, the incidence drops to 1.3.3-5,25
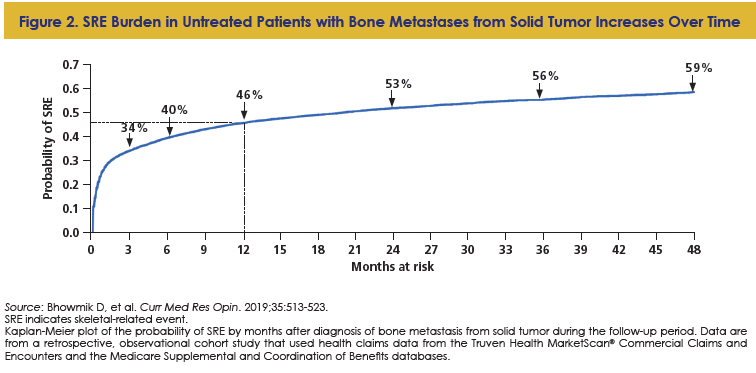
SREs can occur early in the disease course; however, SRE risk does not necessarily go away over time. There is an augmented risk for a subsequent SRE once a first SRE is experienced: in one study, among 343 patients with MM, 119 patients (35%) had a baseline SRE; at a median follow-up of 25.7 months, 34.1% experienced a subsequent SRE.14 Among these patients, 68% of SREs occurred within the first year, and the risk of SREs increased with each relapse.14 With respect to solid tumors, half of treatment-naïve patients with solid tumors and bone metastases experienced an SRE within 2 years following bone metastasis diagnosis (Figure 2).24 Notably, in patients who experience ≥1 SREs, the subsequent SRE may not be the same type as the prior SRE.26
Clinical Impact of Bone Metastases, Osteolytic Lesions, and SREs in Patients with Cancer
Due to tremendous advancements in antitumor treatment efficacy, patients with cancer are living longer. Patients with metastatic disease also benefit from these advancements, and often live for many years after diagnosis.27 Longer survival times have brought into focus the need to manage some cancer as a long-term disease.13 As survival rates improve, the frequency of SREs may also increase, making SRE prevention and management a key component of longer-term care.28 Patients living with SREs due to cancer currently have to tolerate the negative impacts that accompany them. It is important to be aware of the clinical and QOL impacts of bone metastases and osteolytic lesions to provide patient support and education.
Morbidity
SREs cause significant morbidity. Perhaps the most recognizable risk due to bone disease is pathological fractures. Between 9% and 29% of patients with bone metastases will develop pathological fractures, and the risk of fractures is strikingly high for patients with MM.29,30 The spine is a high-risk area for fractures, although long bones are also at risk.29,30
While it is well-understood that bone metastases due to solid tumors and lesions due to MM weaken the bone and make it more prone to fracture, the factors that influence the risk of developing pathological fractures and other SREs remain incompletely understood.29 For patients with solid tumors, risk factors for fractures are thought to include increasing pain, radiographic osteolytic appearance, lesion size >25 mm, axial cortical involvement >30 mm, and circumferential cortical involvement >50%.29 It should be noted, however, that the presence or absence of pain at diagnosis may not be a reliable predictor of SRE risk.31 For patients with MM, body mass index, corticosteroid use, and serum calcium levels are among suggested risk factors for pathological fractures.30,32,33
Patients with other SREs, including spinal cord compression and surgery to bone, also experience reduced QOL and increased pain compared with patients without SREs. Up to 5% of patients dying from cancer develop spinal cord compression, which is severely painful and can quickly render patients bedridden.29,34 Fortunately, outcomes of spinal cord compression, a medical emergency, can be improved if decompressive surgery is performed within 48 hours of the compression event.35 In considering surgery to bone for patients with bone lesions, the risks versus benefits (eg, risk of surgical complications and re-operation rates vs pain relief and functional restoration) associated with surgery should be weighed.36,37 While often used for pain palliation, radiation to bone can benefit patients, but this relief can last <3 months.38
The potential negative impacts of bone involvement are wide-reaching and systemic. For one, bone destruction can be painful, and the resulting hypercalcemia can lead to impaired renal function, gastrointestinal distress, and cognitive and cardiac issues.29 Also, patients with bone metastases and lesions often have reduced mobility, which can lead to complications including deep vein thrombosis and pulmonary complications.39,40 It is clear from these data that bone involvement due to cancer has a negative impact on patients.
Patients with cancer experience survival times that are longer than ever. How should this affect the concern for SREs in patients with bone metastases from cancer or MM?
Jonathan Kaufman, MD: “Addressing bone health in myeloma is a standard part of the initial conversation. It’s certainly something that we discuss. There are pharmacologic measures, but there are also the other health measures and particularly remaining active is important. For remaining active, the recommendation is walking for 30 to 45 minutes most days of the week. For the patients who can’t do that from the start, you just get them to remain active and build up to that goal.”
Tiffany Richards, PhD, MS, APN, AOCNP: “It’s part of the whole treatment plan approach when we’re seeing patients. Obviously, treating the disease is going to hopefully not affect their bones that much, but it is part of the conversation. We want to just keep them healthy.…It’s important for the patients to remain active.”
Beth Faiman, PhD, MSN, APRN-BC, AOCN, FAAN: “I always tell my patients, ‘If you have a new back or bone pain, let us know. It doesn’t necessarily mean that your cancer’s back. We could just proceed with standard treatment.’”
Patient Impact of SREs
As briefly alluded to in the previous section, the negative impact of SREs is not limited to clinical complications. Patients who experience bone involvement due to cancer have undesirable effects, including pain, reduced QOL, worsened mental health, and increased financial burden. These unpleasant consequences affect every aspect of patient life, and it is imperative that physicians remain aware of this component of their patients’ disease.
Pain
In general, pain is a frequent complication for patients with cancer.41 In patients with lesions due to MM and metastases from solid tumors, mechanical factors that underlie the pathogenesis of bone pain include bone destabilization due to lesions and decalcification and mechanosensitive receptor activation, while inflammatory factors, such as nerve stimulation by infiltrating tumor cells, can also trigger pain signaling.16 Among patients with metastatic cancer, 81.4% report bone pain.16 Pain may or may not be present at initial diagnosis.3 Importantly, acute bone pain is often noted as the first symptom of metastasis, but does not always indicate that cancer has spread to bone.23,31,42
Although SREs are often quite painful, bone pain may also not be a reliable predictor for SREs.32 However, SREs have been shown to increase the risk of pain progression and the need for opioids. Clinical guidelines recommend initiating preventive treatment with BTAs at diagnosis of metastatic bone disease or presence of lytic lesions in MM, before signs and symptoms of SREs develop, wherever possible—this presents an opportunity to mitigate or prevent SREs.43
What are best practices for managing patients with SREs?
Beth Faiman, PhD, MSN, APRN-BC, AOCN, FAAN: “The prompt assessment of multiple myeloma symptoms [is important]. Even if we think they are in a nice, stable disease, or at their transplant and we think they should get some good mileage out of that transplant, people will still progress. Being aware of that and having the communication with patients is important.”
Kathleen Colson, RN, BSN, BS: “Fortunately, there are many treatment options for bone complications in cancer patients. Importantly, we understand the need to slow the bone breakdown process. Other treatment best practices to mitigate a patient’s bone pain are the use of over-the-counter medications, opioids, radiation therapy, surgical interventions (ie, kyphoplasty or vertebroplasty), physical therapy, exercise, and, of course, treating their disease. We have to be careful with opioids as they can reduce a patient’s mobility along with other unfavorable side effects (ie, dizziness, lightheadedness, and constipation).”
QOL
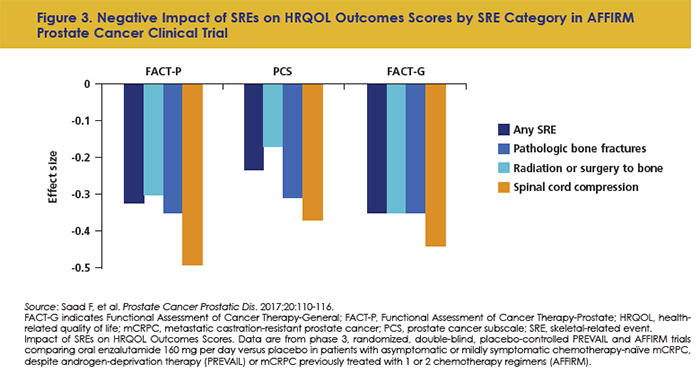
Just as pain is exacerbated by SREs, so is QOL. Pathological fractures are associated with worse QOL and more anxiety and depression in patients with bone metastases.44 In patients with prostate cancer, SREs have been tied to lower Functional Assessment of Cancer Therapy-Prostate (FACT-P) functional well-being scores, higher mean pain severity, and worse pain scores compared with non-SRE patients.45 In an analysis of 2 prostate cancer trials, SREs were tied to a significant functional decline in daily life and health-related QOL (Figure 3).46
SREs can also negatively impact mental health. Symptom uncertainty promotes fear, anxiety, and worry in patients that can be exacerbated by pain.47 Pain disrupts social interaction and interferes with relationships.48 Autonomy and sleep are often disrupted.40,48 In a survey of patients with MM, patients report depression, anxiety, distress, and worrying about the future.49,50 In another study, patients report feeling emotionally vulnerable.51 These data highlight the mental health challenges faced by patients who experience cancer with bone involvement. Physicians should take care to consider the mental health impact of a patient’s disease to inform their interactions with patients and to provide support and compassion as they help patients navigate their disease course.
How do you evaluate QOL in your patients with bone metastases from solid tumors and lesions due to MM?
Beth Faiman, PhD, MSN, APRN-BC, AOCN, FAAN: “In 2015, the Commission on Cancer initiative recommended that everybody should have quality of life evaluated with various measures; they’re institution-specific. When our patients come in, with any tumor type, they get a questionnaire that looks at suicide risk and the risks of depression. If they have a high depression score or they are very, very sad or they are suicidal, they get a call from a social worker who connects patients to support groups and other mental health resources.”
Tiffany Richards, PhD, MS, APN, AOCNP: “If patients have a high level of distress, they’re referred to social work, but we are also asking them questions. Are they having problems eating and drinking? Do they need a nutritionist consult? Do they need physical therapy?
It’s not necessarily a tool, but just watching how they do when they get up from a chair and they go to the exam table can give you a good idea of their mobility. For me, that’s the most useful test that I have to know how mobile they are. If they are having a hard time getting up onto an exam table, then that really signifies to me we’ve got a problem here. How immobile are they at home, and then what the consequences of that are and the social isolation.”
Health Resource Utilization and Increased Costs of Care for Patients with SREs
In addition to the pain, reduced QOL, and emotional consequences, patients who have bone metastases and lytic lesions bear an increased and unexpected financial burden, and patients with SREs have higher healthcare costs than those without.17,49,52-57 Unfortunately, SRE-related hospital charges are increasing.58
Patients with SREs also place a significant burden on the healthcare system. The additional health resource utilization (HRU) by patients with SREs occurs regardless of whether the SREs result from solid tumor metastases or lesions due to MM. For patients with solid tumors, across tumor types, all SRE types are associated with substantial HRU.59 Among 1028 patients with MM, those with SREs had higher healthcare resource utilization compared with those without SREs, and resource utilization and mean total healthcare costs increased with SRE frequency.60 Together, these data provide insight into the increased cost and HRU incurred by patients with SREs.
Support Mechanisms, Tools, and Communication Approaches
Bone involvement due to solid tumors and MM can be difficult for patients to navigate, but physicians, nurses, and other caregivers involved in the treatment of these patients can be a much-needed lifeline. There are ample opportunities for intervention, education, and communication that can be taken advantage of to support patients with bone involvement. The following section details support mechanisms, tools, and communication approaches that can help healthcare providers assess disease burden as it relates to bone involvement and to communicate with their patients.
Imaging
Imaging is of paramount importance in the management of patients with cancer-related bone involvement. Imaging aids in the detection of bone involvement, informs prognosis and treatment decisions, and allows monitoring of patients during follow-up.61 In patients with MM, the detection of lesions influences the timing of treatment initiation.61 There are several imaging approaches available for the assessment of bone involvement, and selection may vary depending on patient factors and the type of lesion to be detected. A computed tomography (CT) scan can detect lytic lesions, but use of CT is limited by radiation toxicity.38,61 Magnetic resonance imaging (MRI) and positron emission tomography (PET) scans can detect focal lesions, although PET/CT is superior.61 Patients with suspected bone disease can be evaluated using an initial low-dose whole-body CT or fluorodeoxyglucose PET/CT.61 Patients with negative or inconclusive full-body CT results should proceed to evaluation with axial or whole-body MRI.61 Patients with negative results should receive yearly follow-up to monitor for developing bone disease.61
Unmet needs for SRE prevention
Many patients with cancer experience SREs before BTA therapy is initiated. According to a 2015 analysis of Medicare data, >50% of patients with solid tumors and SREs did not initiate treatment with BTAs until after they had experienced ≥1 events.62 In addition, patients who are on BTA treatment may be removed for various reasons, including disease progression and lack of perceived patient compliance.63 These real-world data highlight an unmet need for efficient deployment of BTAs in patients who need them.
In patients with solid tumors, BTAs should be initiated as soon as bone metastases are detected and should be continued throughout the disease course.43 In patients with MM, those with evidence of lytic disease should also receive treatment with BTAs.64 Clinicians should monitor patients receiving BTAs for renal issues, hypocalcemia, and osteonecrosis of the jaw.64 A dental examination and preventive dentistry are recommended for all patients with cancer prior to initiation of BTA treatment.64
What factors into your decision-making process when selecting treatment for a patient with an SRE?
Beth Faiman, PhD, MSN, APRN-BC, AOCN, FAAN: “An existing skeletal-related event is a risk factor for future development. Once they get a compression fracture, that begets more and more…unless you protect them with the BTAs, good disease control, and exercise.”
Personalized medicine
Individualized treatment is key to managing pain, optimizing outcomes, and supporting QOL in patients with cancer.16 Treatment decisions for patients with SREs related to solid tumors or MM should consider many factors, including age, fitness, and length of treatment.16,65 Health-related QOL factors, including patient goals, priorities, and expectations, should also be taken into account when making treatment decisions in a shared decision-making setting.66 Patient considerations may vary with age, and as SREs add to the cost of care, attention should be paid to this factor as it is a burden to patients and can influence treatment adherence.52,67 Patients may also prioritize social interaction; these patients may find it difficult to cope with disease or adhere to treatment regimens that interfere with travel and socializing.51 Caregivers are also an important component of a patient’s care and should be involved in the receipt of education and support.68
Multidisciplinary management
Optimal care for patients with SREs requires a multidisciplinary management approach.66,69 Patients with bone involvement require different types of support for both preventive treatment and treatment for existing bone disease or SREs, including imaging, therapeutics, and social support. Oncologists, orthopedic surgeons, specialist nurses, and interventional radiologists may all play a role in managing these patients; specialists in palliative medicine and symptom control may also provide helpful input.69,70
Assessment tools
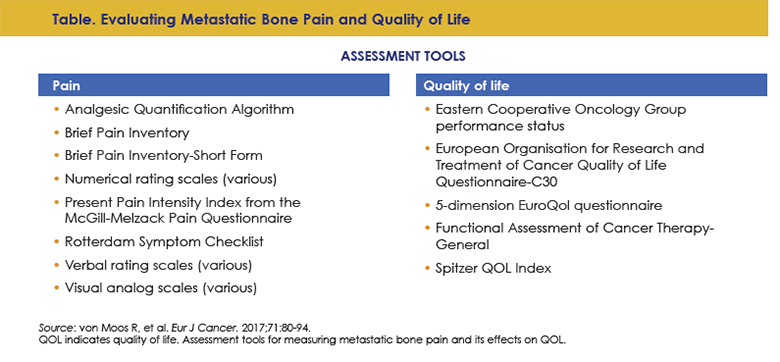
Careful assessment is also an important component of care that can help healthcare providers track disease burden, determine fitness, and understand the QOL impact of a given patient’s disease. Thorough patient evaluation is an important component of caring for patients with and at risk for SREs.16 While there is no tool available to predict a patient’s risk of developing an SRE, existing pain assessment tools, including numerical and verbal rating scales, and QOL assessment tools, including the Eastern Cooperative Oncology Group performance status and FACT score, among several others (Table), can help healthcare providers gain an accurate picture of an individual patient’s disease and symptom burden regarding bone involvement.16 Asking specific questions can also help with assessment.68
Communication and education
Communication and education are both important components of treating all patients and present opportunities to reassure, dispel fears, and answer questions. In fact, patients report that their reassurance depends on the information they receive from their healthcare provider about their disease.51 Thus, clinicians should provide patient education to support their patients with cancer and bone involvement.68 Clinicians should discuss disease biology, available testing, symptom and adverse event management, and patient and caregiver emotional support.68
Despite the importance of communication, some patients report needing more information from their providers on cancer bone health. In a 2016 survey of 557 patients with MM, one of the most burdensome problems reported was information need. Among these patients, 29.4% said that they did not have enough information about their risk and what might happen in the future.71
In addition to disease education, communication with clinicians can help patients cope with their disease and the associated negative factors. For example, healthcare providers can offer advice and encouragement for the use of self-care strategies, or connect patients with funding resources.72 Physicians can also help guide beneficial lifestyle changes by providing information on safe exercise, supportive nutrition, and smoking cessation.70 A survivorship care plan is a useful tool that can help guide patients and clinicians through discussions about patient desires and the goals and expectations of therapy (Figure 4).73 Together, purposeful communication and patient education can help patients feel better about their disease, can encourage compliance, and can provide important information to healthcare providers to provide the best possible care.
How do you counsel patients with solid tumors or MM and bone disease?
Beth Faiman, PhD, MSN, APRN-BC, AOCN, FAAN: “Education is key when you’re talking about bone health. Any new back or bone pain, any new problems of leg weakness or incontinence, let us know. What I go back to is educating about the pathobiology of the disease.”
Kathleen Colson, RN, BSN, BS: “We’re having conversations with our patients….We see these patients all the time. We have very close relationships with them.”
How do you help your patients understand their diagnoses?
Jonathan Kaufman, MD: “My approach is that, especially with the new patients, I spend the majority of the time on the overall disease, and then we go through bone-related issues, infection-related issues, blood clotting–related issues, anemia, and try to be very structured in the communication and provide notes so that the communication doesn’t end with me talking to the patient.”
Kathleen Colson, RN, BSN, BS: “Everybody is overwhelmed when they receive a cancer diagnosis. Education is key to helping patients understand their disease. Also, setting expectations of treatment—letting patients know they will most likely be on treatment the remainder of their life. I believe in giving patients educational information about their disease and their treatment regimen in advance and they can read it at home when they are not involved in the highly stressful situation.”
How do you reassure your patients with bone pain?
Beth Faiman, PhD, MSN, APRN-BC, AOCN, FAAN: “Obviously, if they have an acute event, we try to jump in and treat it promptly. That’s the reassurance I provide; if I can control the tumor, we’ll oftentimes improve your pain. Sometimes we need radiation to help. Sometimes we need surgery to help. I underscore the importance of the multiteam approach.”
Tiffany Richards, PhD, MS, APN, AOCNP: “I also think sending them to supportive care can be really helpful, because that psychosocial piece I think is really, really key for patients. Then, reassuring them that it is OK for them to start getting up and moving again. Sometimes there’s a hesitancy, because they’re afraid they’re going to fall, and so they don’t want to move. Trying to provide that reassurance to them so that they can start moving.”
How do you support mental health in your patients with bone disease?
Beth Faiman, PhD, MSN, APRN-BC, AOCN, FAAN: “There are patient support groups where people can go, and they can talk to other people with their tumor types, and participate in functional medicine kinds of things—they can take tai chi and reiki and get massages.”
Kathleen Colson, RN, BSN, BS: “At Dana-Farber, we have a multiple myeloma support group for patients that meets once a month. Also, in the community, there are big organizations out there that organize support group meetings for these patients, too. I called a patient the other day and her husband said, ‘Oh, she’s on her Zoom call for her myeloma support group.’ Everybody finds a way.”
Conclusion
Thanks to tremendous advancements in cancer treatment, patients have seen great leaps in overall survival, including those with metastatic disease.27 Unfortunately, most patients with cancer metastasis to bone or lytic lesions will develop SREs during the course of their disease.7,11
References
- Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 2008;3(Suppl 3):S131-S139.
- Li S, Peng Y, Weinhandl ED, et al. Estimated number of prevalent cases of metastatic bone disease in the US adult population. Clin Epidemiol. 2012;4:87-93.
- Lipton A, Theriault RL, Hortobagyi GN, et al. Pamidronate prevents skeletal complications and is effective palliative treatment in women with breast carcinoma and osteolytic bone metastases: long term follow-up of two randomized, placebo-controlled trials. Cancer. 2000;88:1082-1090.
- Rosen LS, Gordon D, Tchekmedyian NS, et al. Long-term efficacy and safety of zoledronic acid in the treatment of skeletal metastases in patients with nonsmall cell lung carcinoma and other solid tumors: a randomized, phase III, double-blind, placebo-controlled trial. Cancer. 2004;100:2613-2621.
- Saad F, Gleason DM, Murray R, et al. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst. 2004;96:879-882.
- Macedo F, Ladeira K, Pinho F, et al. Bone metastases: an overview. Oncol Rev. 2017;11:321.
- Hernandez RK, Wade SW, Reich A, et al. Incidence of bone metastases in patients with solid tumors: analysis of oncology electronic medical records in the United States. BMC Cancer. 2018;18:44.
- Selvaggi G, Scagliotti GV. Management of bone metastases in cancer: a review. Crit Rev Oncol Hematol. 2005;56:365-378.
- Wang C, Shen Y, Zhu S. Distribution features of skeletal metastases: a comparative study between pulmonary and prostate cancers. PLoS One. 2015;10:e0143437.
- Wang CY, Wu GY, Shen MJ, et al. Comparison of distribution characteristics of metastatic bone lesions between breast and prostate carcinomas. Oncol Lett. 2013;5:391-397.
- Silbermann R, Roodman GD. Myeloma bone disease: pathophysiology and management. J Bone Oncol. 2013;2:59-69.
- Oster G, Lamerato L, Glass AG, et al. Natural history of skeletal-related events in patients with breast, lung, or prostate cancer and metastases to bone: a 15-year study in two large US health systems. Support Care Cancer. 2013;21:3279-3286.
- von Moos R, Body JJ, Egerdie B, et al. Pain and analgesic use associated with skeletal-related events in patients with advanced cancer and bone metastases. Support Care Cancer. 2016;24:1327-1337.
- Kim C, Bhatta S, Cyprien L, et al. Incidence of skeletal-related events among multiple myeloma patients in the United States at oncology clinics: observations from real-world data. J Bone Oncol. 2019;14:100215.
- FDA. Clinical trial endpoints for the approval of cancer drugs and biologics: guidance for industry. Updated December 2018. www.fda.gov/media/71195/download. Accessed July 23, 2021.
- von Moos R, Costa L, Ripamonti CI, et al. Improving quality of life in patients with advanced cancer: targeting metastatic bone pain. Eur J Cancer. 2017;71:80-94.
- Usmanova E, Shchelkova O, Sushentsov E, Valiev A. Quality of life and attitude to disease in patients with bone tumors. Sleep Hypn. 2019;21:51-59.
- Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004;350:1655-1664.
- Seeman E, Delmas PD. Bone quality—the material and structural basis of bone strength and fragility. N Engl J Med. 2006;354:2250-2261.
- Yaccoby S. Advances in the understanding of myeloma bone disease and tumour growth. Br J Haematol. 2010;149:311-321.
- Croucher PI, McDonald MM, Martin TJ. Bone metastasis: the importance of the neighbourhood. Nat Rev Cancer. 2016;16:373-386.
- Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2:584-593.
- Park SH, Eber MR, Widner DB, Shiozawa Y. Role of the bone microenvironment in the development of painful complications of skeletal metastases. Cancers (Basel). 2018;10:141.
- Bhowmik D, Song X, Intorcia M, et al. Examination of burden of skeletal-related events in patients naive to denosumab and intravenous bisphosphonate therapy in bone metastases from solid tumors population. Curr Med Res Opin. 2019;35:513-523.
- Berenson JR, Lichtenstein A, Porter L, et al. Long-term pamidronate treatment of advanced multiple myeloma patients reduces skeletal events. Myeloma Aredia Study Group. J Clin Oncol. 1998;16:593-602.
- Abdulhalim AM, Hussain A, Mullins CD, et al. Burden and timing of first and subsequent skeletal related events (SREs) in United States elderly men with metastatic prostate cancer. Value Health. 2014;17:72.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30.
- Pruksakorn D, Phanphaisarn A, Settakorn J, et al. Prognostic score for life expectancy evaluation of lung cancer patients after bone metastasis. J Bone Oncol. 2018;10:1-5.
- Tsuzuki S, Park SH, Eber MR, et al. Skeletal complications in cancer patients with bone metastases. Int J Urol. 2016;23:825-832.
- Melton LJ 3rd, Kyle RA, Achenbach SJ, et al. Fracture risk with multiple myeloma: a population-based study. J Bone Miner Res. 2005;20:487-493.
- Saad F, Eastham J. Zoledronic acid improves clinical outcomes when administered before onset of bone pain in patients with prostate cancer. Urology. 2010;76:1175-1181.
- Chen YL, Liu YC, Wu CH, et al. Role of BMI and age in predicting pathologic vertebral fractures in newly diagnosed multiple myeloma patients: a retrospective cohort study. Hematol Oncol. 2018;36:407-415.
- Xiao R, Miller JA, Margetis K, et al. Predicting the progression of vertebral fractures in patients with multiple myeloma. Spine J. 2016;16:510-515.
- Lawton AJ, Lee KA, Cheville AL, et al. Assessment and management of patients with metastatic spinal cord compression: a multidisciplinary review. J Clin Oncol. 2019;37:61-71.
- Fürstenberg CH, Wiedenhöfer B, Gerner HJ, Putz C. The effect of early surgical treatment on recovery in patients with metastatic compression of the spinal cord. J Bone Joint Surg Br. 2009;91:240-244.
- Ratasvuori M, Wedin R, Keller J, et al. Insight opinion to surgically treated metastatic bone disease: Scandinavian Sarcoma Group Skeletal Metastasis Registry report of 1195 operated skeletal metastasis. Surg Oncol. 2013;22:132-138.
- Weiss RJ, Tullberg E, Forsberg JA, et al. Skeletal metastases in 301 breast cancer patients: patient survival and complications after surgery. Breast. 2014;23:286-290.
- Gralow JR, Biermann JS, Farooki A, et al. NCCN Task Force report: bone health in cancer care. J Natl Compr Canc Netw. 2009;7(Suppl 3):S1-S32.
- Coluzzi F, Rolke R, Mercadante S. Pain management in patients with multiple myeloma: an update. Cancers (Basel). 2019;11:2037.
- Baz R, Lin HM, Hui AM, et al. Development of a conceptual model to illustrate the impact of multiple myeloma and its treatment on health-related quality of life. Support Care Cancer. 2015;23:2789-2797.
- van den Beuken-van Everdingen MH, Hochstenbach LM, Joosten EA, et al. Update on prevalence of pain in patients with cancer: systematic review and meta-analysis. J Pain Symptom Manage. 2016;51:1070-1090.e1079.
- Mantyh P. The science behind metastatic bone pain. Eur J Cancer. 2006;4:4-8.
- Coleman R, Hadji P, Body JJ, et al. Bone health in cancer: ESMO clinical practice guidelines. Ann Oncol. 2020;31:1650-1663.
- van der Vliet QM, Paulino Pereira NR, Janssen SJ, et al. What factors are associated with quality of life, pain interference, anxiety, and depression in patients with metastatic bone disease? Clin Orthop Relat Res. 2017;475:498-507.
- McKay R, Haider B, Duh MS, et al. Impact of symptomatic skeletal events on health-care resource utilization and quality of life among patients with castration-resistant prostate cancer and bone metastases. Prostate Cancer Prostatic Dis. 2017;20:276-282.
- Saad F, Ivanescu C, Phung D, et al. Skeletal-related events significantly impact health-related quality of life in metastatic castration-resistant prostate cancer: data from PREVAIL and AFFIRM trials. Prostate Cancer Prostatic Dis. 2017;20:110-116.
- Heathcote LC, Eccleston C. Pain and cancer survival: a cognitive-affective model of symptom appraisal and the uncertain threat of disease recurrence. Pain. 2017;158:1187-1191.
- Minello C, George B, Allano G, et al. Assessing cancer pain-the first step toward improving patients’ quality of life. Support Care Cancer. 2019;27:3095-3104.
- Ramsenthaler C, Kane P, Gao W, et al. Prevalence of symptoms in patients with multiple myeloma: a systematic review and meta-analysis. Eur J Haematol. 2016;97:416-429.
- Kiely F, Cran A, Finnerty D, O’Brien T. Self-reported quality of life and symptom burden in ambulatory patients with multiple myeloma on disease-modifying treatment. Am J Hosp Palliat Care. 2017;34:671-676.
- Mortensen G, Salomo M. Quality of life in patients with multiple myeloma: a qualitative study. J Cancer Sci Ther. 2016;8:289-293.
- Huntington SF, Weiss BM, Vogl DT, et al. Financial toxicity in insured patients with multiple myeloma: a cross-sectional pilot study. Lancet Haematol. 2015;2:e408-e416.
- Bhowmik D, Hines DM, Karkare S, et al. Incremental healthcare resource use and costs associated with skeletal-related events in patients with bone metastases from solid tumors. Poster presented at ISPOR 23rd Annual International Meeting. Baltimore, Maryland, May 19-23, 2018. Abstract PHS32.
- Delea T, McKiernan J, Brandman J, et al. Retrospective study of the effect of skeletal complications on total medical care costs in patients with bone metastases of breast cancer seen in typical clinical practice. J Support Oncol. 2006;4:341-347.
- McDougall JA, Bansal A, Goulart BH, et al. The clinical and economic impacts of skeletal-related events among Medicare enrollees with prostate cancer metastatic to bone. Oncologist. 2016;21:320-326.
- Ailawadhi S, Medhekar R, Princic N, et al. Healthcare resource utilization and costs in patients with multiple myeloma with and without skeletal-related events. J Oncol Pharm Pract. 2020;26:1070-1079.
- Bhowmik D, Hines DM, Intorcia M, Wade RL. Economic burden of skeletal-related events in patients with multiple myeloma: analysis of US commercial claims database. J Med Econ. 2018;21:622-628.
- Roghmann F, Antczak C, McKay RR, et al. The burden of skeletal-related events in patients with prostate cancer and bone metastasis. Urol Oncol. 2015;33:17.e19-17.e18.
- Mahmood A, Ghazal H, Fink MG, et al. Health-resource utilization attributable to skeletal-related events in patients with advanced cancers associated with bone metastases: results of the US cohort from a multicenter observational study. Community Oncol. 2012;9:148-157.
- Nash Smyth E, Conti I, Wooldridge JE, et al. Frequency of skeletal-related events and associated healthcare resource use and costs in US patients with multiple myeloma. J Med Econ. 2016;19:477-486.
- Hillengass J, Usmani S, Rajkumar SV, et al. International myeloma working group consensus recommendations on imaging in monoclonal plasma cell disorders. Lancet Oncol. 2019;20:e302-e312.
- Hernandez RK, Adhia A, Wade SW, et al. Prevalence of bone metastases and bone-targeting agent use among solid tumor patients in the United States. Clin Epidemiol. 2015;7:335-345.
- Henry D, von Moos R, Body JJ, et al. Bone-targeted agent treatment patterns and the impact of bone metastases on patients with advanced breast cancer in the United States. Curr Med Res Opin. 2019;35:375-381.
- Anderson K, Ismaila N, Flynn PJ, et al. Role of bone-modifying agents in multiple myeloma: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2018;36:812-818.
- Jordan K, Proskorovsky I, Lewis P, et al. Effect of general symptom level, specific adverse events, treatment patterns, and patient characteristics on health-related quality of life in patients with multiple myeloma: results of a European, multicenter cohort study. Support Care Cancer. 2014;22:417-426.
- Seitzler S, Finley-Oliver E, Simonelli C, Baz R. Quality of life in multiple myeloma: considerations and recommendations. Expert Rev Hematol. 2019;12:419-424.
- Tran G, Zafar SY. Financial toxicity and implications for cancer care in the era of molecular and immune therapies. Ann Transl Med. 2018;6:166.
- Rygiel KA, Drozd M, Bułaś L. Care of cancer patients with liver and bone metastases - the place of pharmaceutical care in a balanced plan, focused on the patient’s needs and goals. Arch Med Sci. 2017;13:1483-1492.
- Kimura T. Multidisciplinary approach for bone metastasis: a review. Cancers (Basel). 2018;10:156.
- Drudge-Coates L, van Muilekom E, de la Torre-Montero JC, et al. Management of bone health in patients with cancer: a survey of specialist nurses. Support Care Cancer. 2020;28:1151-1162.
- Ramsenthaler C, Osborne TR, Gao W, et al. The impact of disease-related symptoms and palliative care concerns on health-related quality of life in multiple myeloma: a multi-centre study. BMC Cancer. 2016;16:427.
- Cormican O, Dowling M. Living with relapsed myeloma: symptoms and self-care strategies. J Clin Nurs. 2018;27:1713-1721.
- Kurtin S. Living with multiple myeloma: a continuum-based approach to cancer survivorship. Semin Oncol Nurs. 2017;33:348-361.